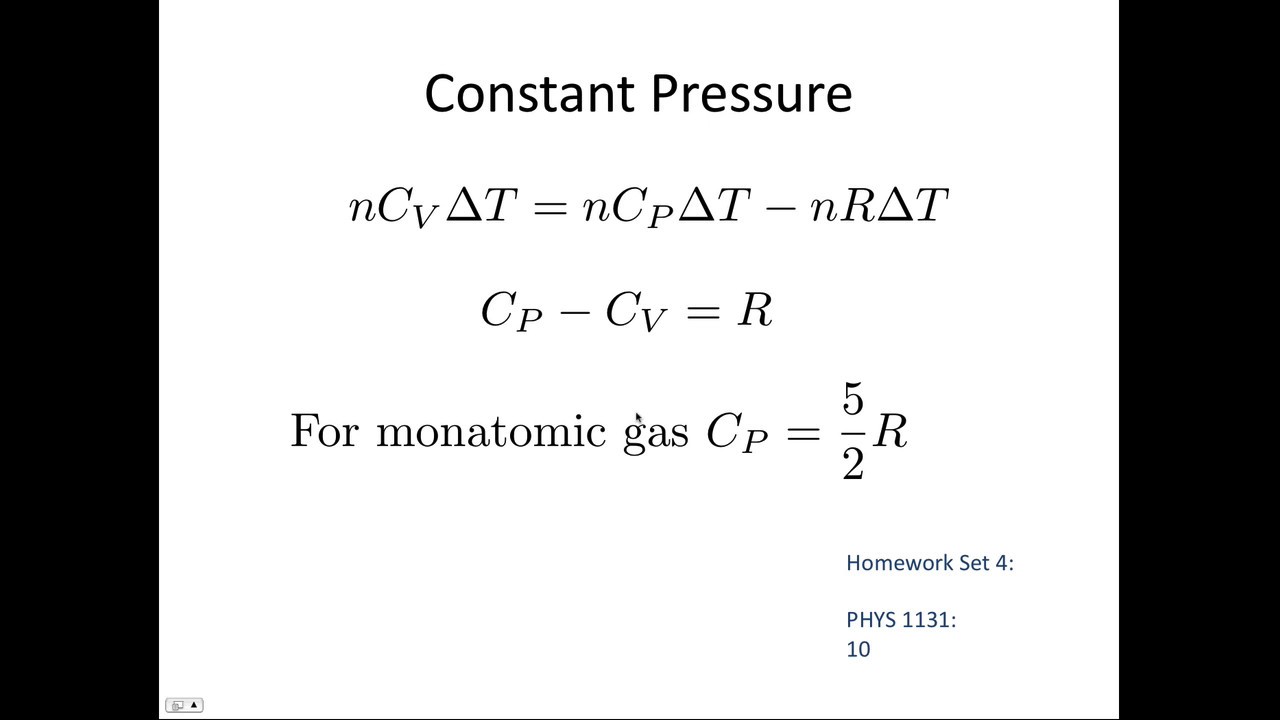
The ratio of the two specific heats is called the adiabatic ratio of the gas. The Specific Heat Capacity is measured and reported at constant pressure Cp or constant volume Cv conditions.
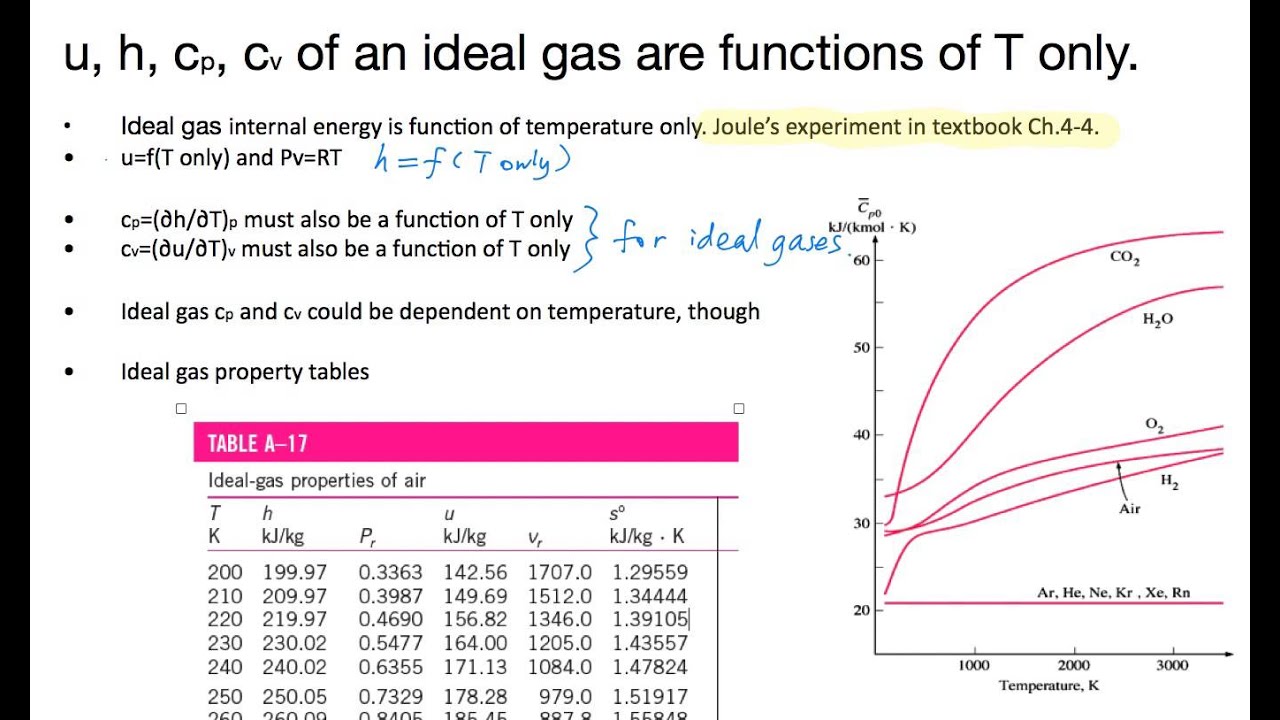 Cp And Cv Of Air Best Resume Examples
Cp And Cv Of Air Best Resume Examples
The purpose of this study is to determine the value of the heat capacity ratio γ CpCV for giving gases such as argon oxygen nitrogen and nitrous oxide using adiabatic expansion.
Cp/cv ratio for gases. Now very quickly pop the stopper from the bottleneck and return it very quickly to its original position Figure 2. This ratio is used to define 1 adiabatic process pVg const and 2 speed of sound in gases v p gRTM. Ratio of Specific Heat The Ratio of Specific Heat can be expressed as.
The ratio C P C V is given by γ C P C V 1 2 n where n is the number of degrees of freedom. Substitute values to get ΔT B CpCvΔT A 42 K. Now you begin with the gas at atmospheric pressure 760 Torr and then add gas to increase the pressure inside the bottle by a small amount say 15 114 Torr.
Relation between C P and C V for ideal gases. Its value for air is 14. 332010 Ive learned that Cp Cv R where R is the gas constant and that Cv 3R2 for a monatomic gas 5R2 for a diatomic gas and that Cp 5R2 for a monatomic gas 7R2 for a diatomic gas but have been unable to find where these numbers come from.
Specific Heat at Const. Since the number of degrees of freedom for monatomic gas 3 is less than that of diatomic gas 5 γ m o n a t o m i c. Using the definition of enthalpy h u Pv and writing the differential of enthalpy the relationship between the specific heats for ideal gases is.
49 rows The specific heat specific heat capacity at constant pressure and constant volume. Setup for measuring the ratio of CpCv for gases. For monoatomic gases Cp25Ru JmolK and Cv15Ru JmolK repectively.
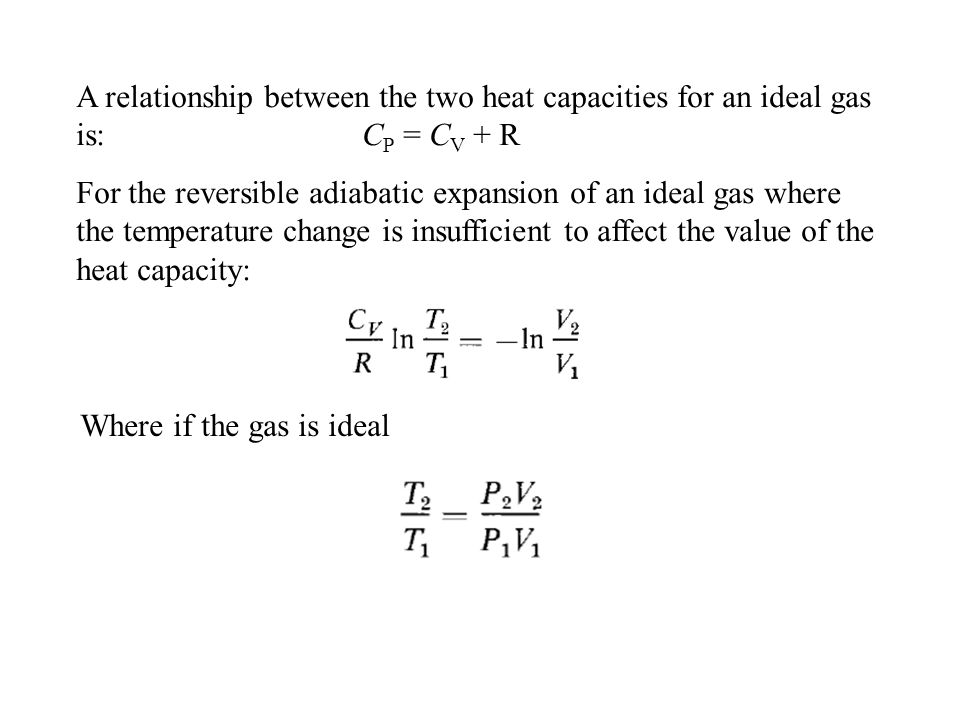
The ratio of the specific heats also called adiabatic index is given by g Cp Cv 1 2 f. Critical temperature Tc. Ii Cp Cv nR and this equation applies for ideal gases.
Properties of Various Ideal Gases at 300 K Gas. Where R is the particular gas constant. Specific Heat at Const.
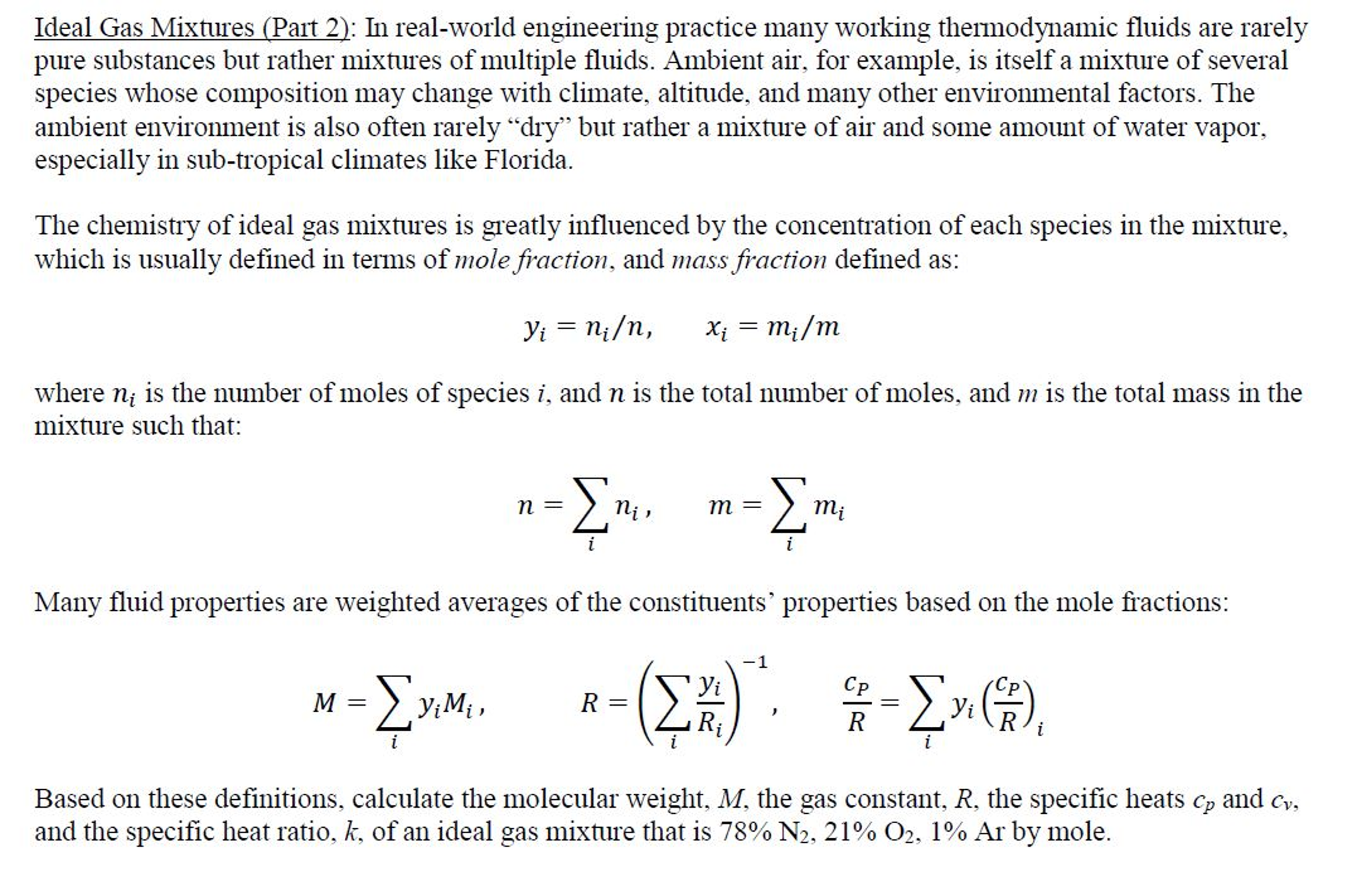
Critical Pressure Pc. Two moles of an ideal gas with CPCV 53 are mixed with 3 moles of another ideal gas with CPCV 43. Accentric Factor ω.
γ d i a t o m i c Questions from AIIMS 1998. Ideal gas specific heat constants Cp IG. By SuttonBiblarz is to use the molar values for Cp and Cv.
The difference cp - cv is constant for an ideal gas. It is the ratio of two specific heat capacities Cp and Cv is given by. The Heat Capacity at Constant Pressure Cp Heat capacity at Constant Volume Cv The isentropic expansion factor is another name for heat capacity ratio that is also denoted for an ideal gas by γ gamma.
8222014 The expansion method uses the clement and desormes method to determine the cpcv ratio for gases. 582009 The third way according to prexs answer confirmed by Rocket Propulsion Elements. The heat given to the two gases is equal QA QB Q A Q B.
Mkgkmol RkJkgK CpkJkgK CvkJkgK k CpCv. For an ideal gas CP CV R whereby the values of CP and CV represent the molar heat capacities at constant pressure and volume. Critical constants for Methane are as following.
Expresses the heat capacity the heat energy required to change a temperature by a certain amount of a gas held at a constant pressure C V the heat capacity of a gas held at constant volume. KCpmix Cpmix - R Cpmix 32 x 20786 68 2938 2663 Jkgmol. Problem from IIT JEE 1990.
The ratio of specific heats for a diatomic gas is CpCv 75 C p C v 7 5. Υ 1 f 2. The specific heat ratio k fluids texts often use g instead of k is defined as.
To view the ratio which I usually call the adiabatic index as a measure of the degrees of freedom of the gas. Calculate specific heat ratio γ CpCv for methane gas at 11 Bar. Cp Cv Ratio for Monoatomic Diatomic and Triatomic Gases Gases can further be classified in terms of the atomicity of its molecules Atomicity is the total number of atoms present in one molecule of an element compound or a substance For example in Oxygen O2.
Δ T B C p C v Δ T A 42 K. The ratio of the specific heats is 53 for monatomic ideal gas and 75 for diatomic gas. The reliability of this experiment is good with a confidence level of up to 95.
This experiment is mainly based on measuring the pressure of the gases. K cp cv 8. In general CpCv a 2 TVK T where a is the expansion coefficient and K T is the isothermal compressibility.
 334 Me Gusta 0 Comentarios Chavan S Education Hub Thechavanclasses En Instagram Specific Heat Formulae Do Follow Thermodynamics Physics Latent Heat
334 Me Gusta 0 Comentarios Chavan S Education Hub Thechavanclasses En Instagram Specific Heat Formulae Do Follow Thermodynamics Physics Latent Heat
Cp And Cv Of Air Best Resume Examples
 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
 Heat Capacity Ratios Of Gases Ppt Video Online Download
Heat Capacity Ratios Of Gases Ppt Video Online Download
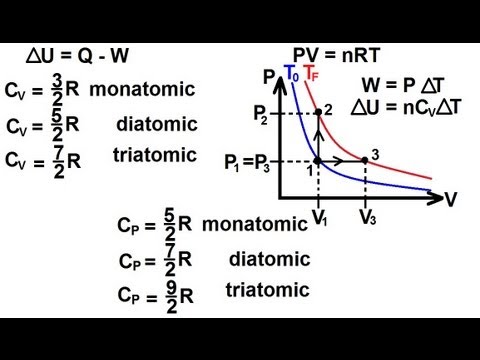 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
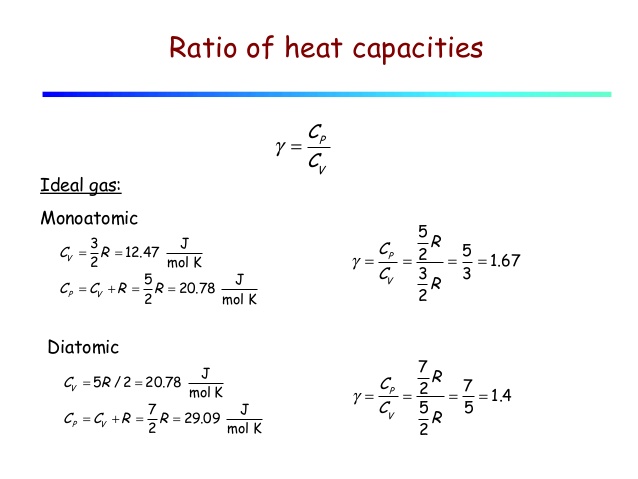 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
 Aerospike Nozzle And Minimum Length Nozzle Design Nozzle Design Gas Constant Nozzle
Aerospike Nozzle And Minimum Length Nozzle Design Nozzle Design Gas Constant Nozzle
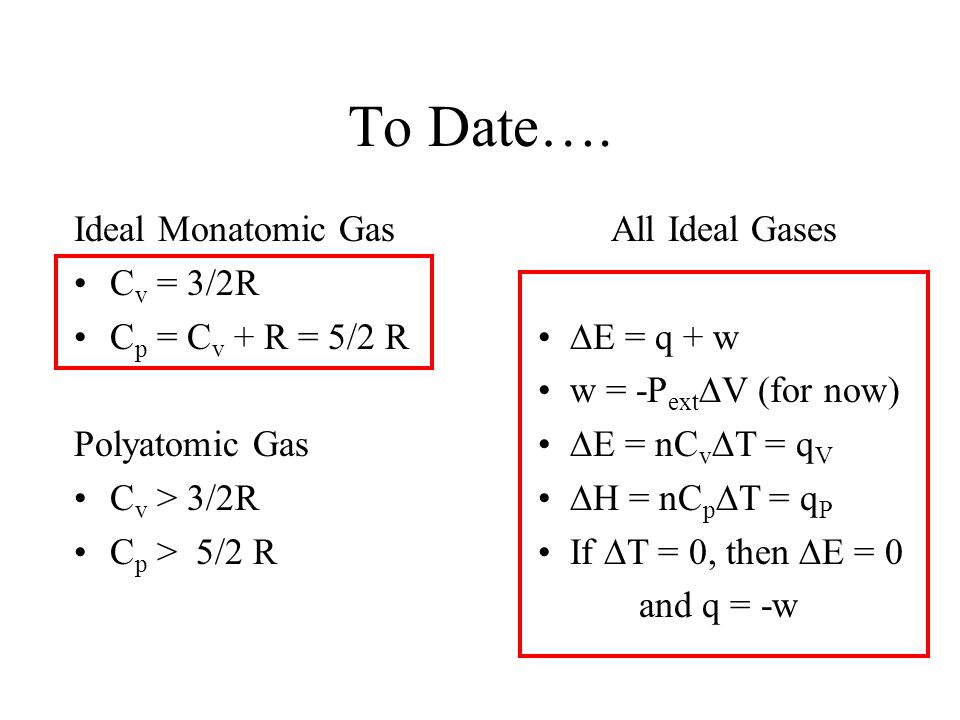 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
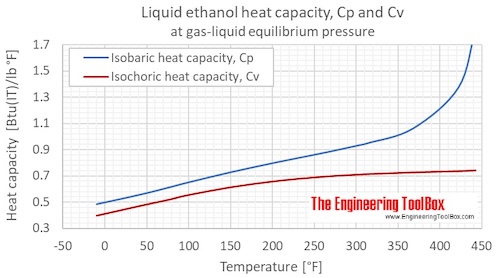 Ethanol Specific Heat C Sub P Sub And C Sub V Sub
Ethanol Specific Heat C Sub P Sub And C Sub V Sub
Cp And Cv Of Water Best Resume Examples
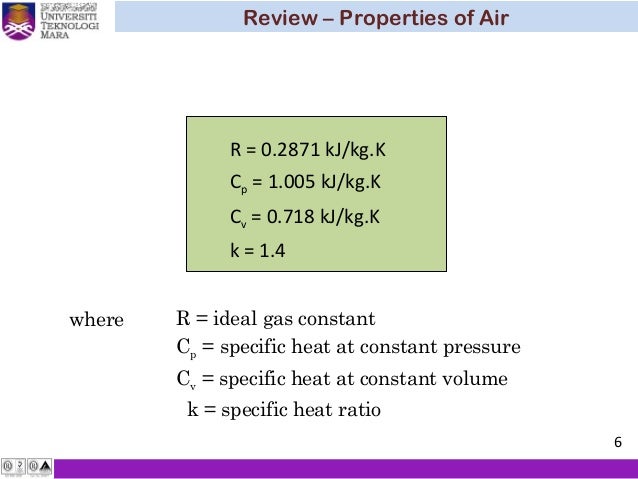 Cp And Cv Of Air Best Resume Examples
Cp And Cv Of Air Best Resume Examples
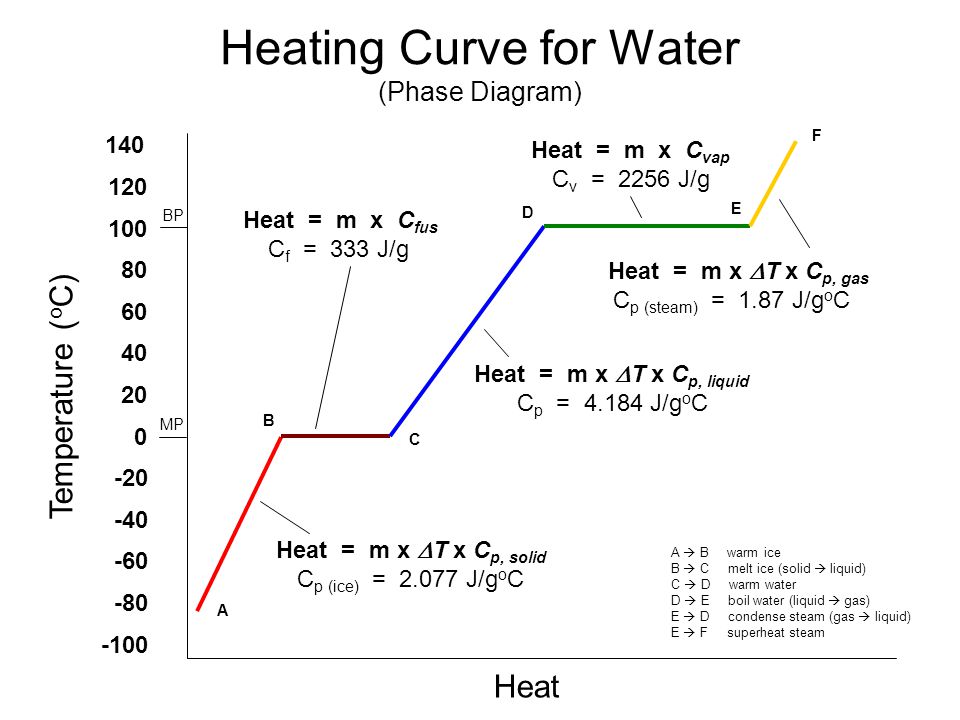 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
 Translational Partition Function Of A Particle Confined In A Box Thermodynamics Mechanical Energy Physical Chemistry
Translational Partition Function Of A Particle Confined In A Box Thermodynamics Mechanical Energy Physical Chemistry
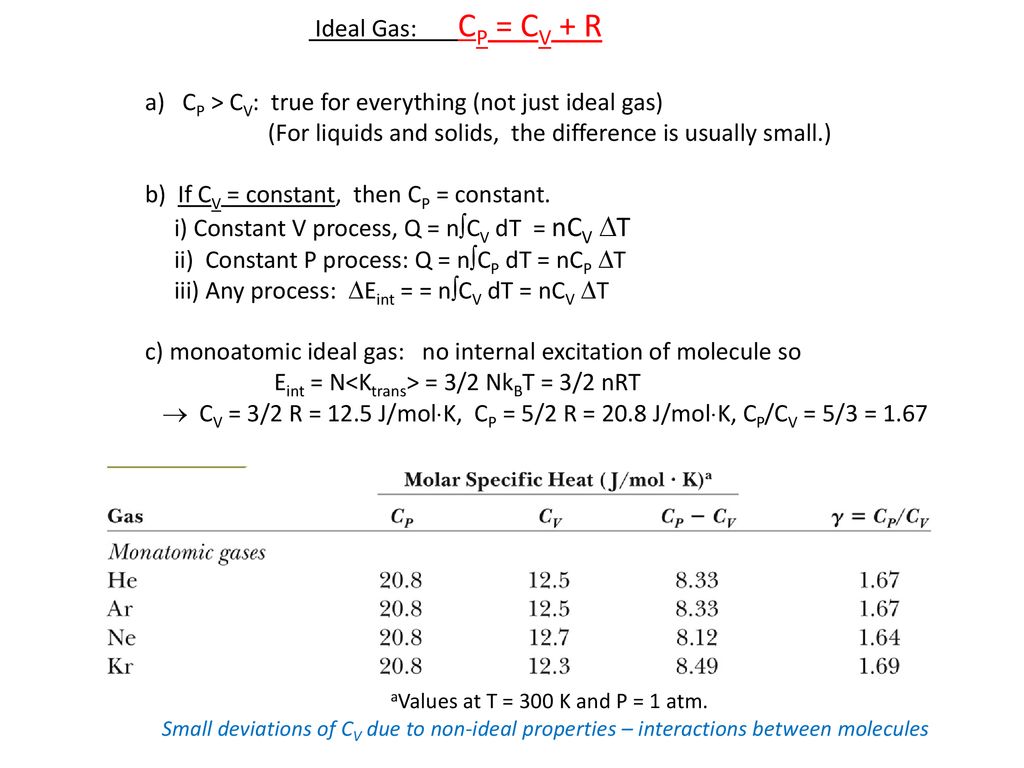 Molar Specific Heat Of Ideal Gases Ppt Download
Molar Specific Heat Of Ideal Gases Ppt Download
Variation Of Ideal Gas Heat Capacity Ratio With Temperature And Relative Density Campbell Tip Of The Month
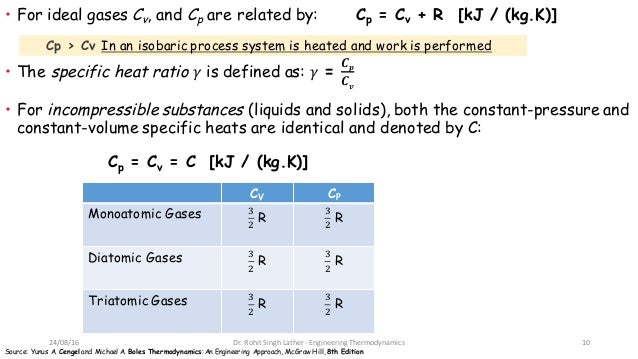 Cp And Cv Of Water Best Resume Examples
Cp And Cv Of Water Best Resume Examples
Variation Of Natural Gas Heat Capacity With Temperature Pressure And Relative Density Campbell Tip Of The Month
 Cp And Cv Of Air Best Resume Examples
Cp And Cv Of Air Best Resume Examples